This market research study involved the extensive use of secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and financial study of the global biologics safety testing market.
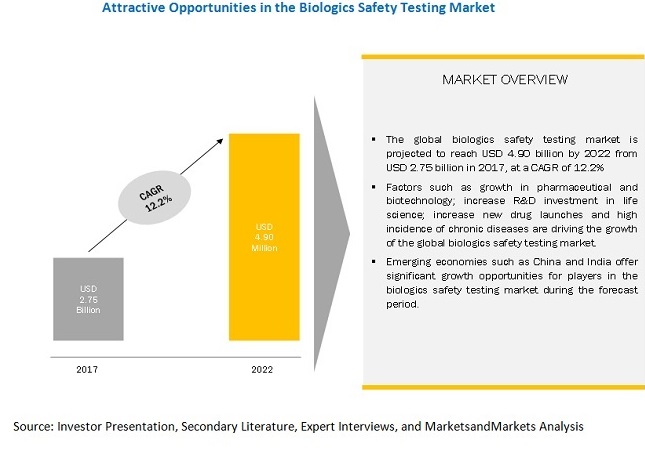
[185 Pages Report] The global biologics safety testing market is projected to reach USD 6.2 billion by 2026 from USD 3.4 billion in 2021, at a CAGR of 12.4% during the forecast period.
The growth of the global biologics safety testing market is driven by factors such as the growth in the biologics and biosimilars markets, growing concerns over cell culture contamination, and rising biopharmaceuticals R&D activities and investments. In addition, emerging markets and increasing biopharmaceutical outsourcing are expected to provide significant growth opportunities for players in the Biologics safety testing market.
Market Dynamics
- Growth in the biosimilars and biologics markets
- Growing concerns over cell culture contamination
- Rising biopharmaceuticals R&D activities and investments
- Emerging markets offer lucrative opportunities
- Increasing biopharmaceutical outsourcing
Many companies are investing heavily in the development of biologics and biosimilars. Currently, more than half of the drug candidates in the discovery stage are biologics, such as proteins, peptides, and monoclonal antibodies. Biologics are expected to contribute around half of the revenue generated by the top 100 pharmaceutical products in the coming years.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=34624144
Based on application, the biologics safety testing market is segmented into monoclonal antibodies manufacturing, vaccines manufacturing, blood and blood products manufacturing, cellular and gene therapy products manufacturing, and other applications. In 2020, the monoclonal antibodies manufacturing segment accounted for the largest market share. The large share of this segment can be attributed to the rising prevalence of diseases and increasing government initiatives for the development of monoclonal antibody drugs.
The geographical regions mapped in the report are:
North America accounted for the largest share of the biologics safety testing market in 2020. Rapid growth in the biopharmaceutical industry is the major factor driving the growth of the North American biologics safety testing market. Growing academic and government investments, rising demand for high-quality research tools for data reproducibility, increasing awareness among consumers about product safety, and the presence of major market players in this region are also driving the market for biologics safety testing in North America.

 Log in with Facebook
Log in with Facebook