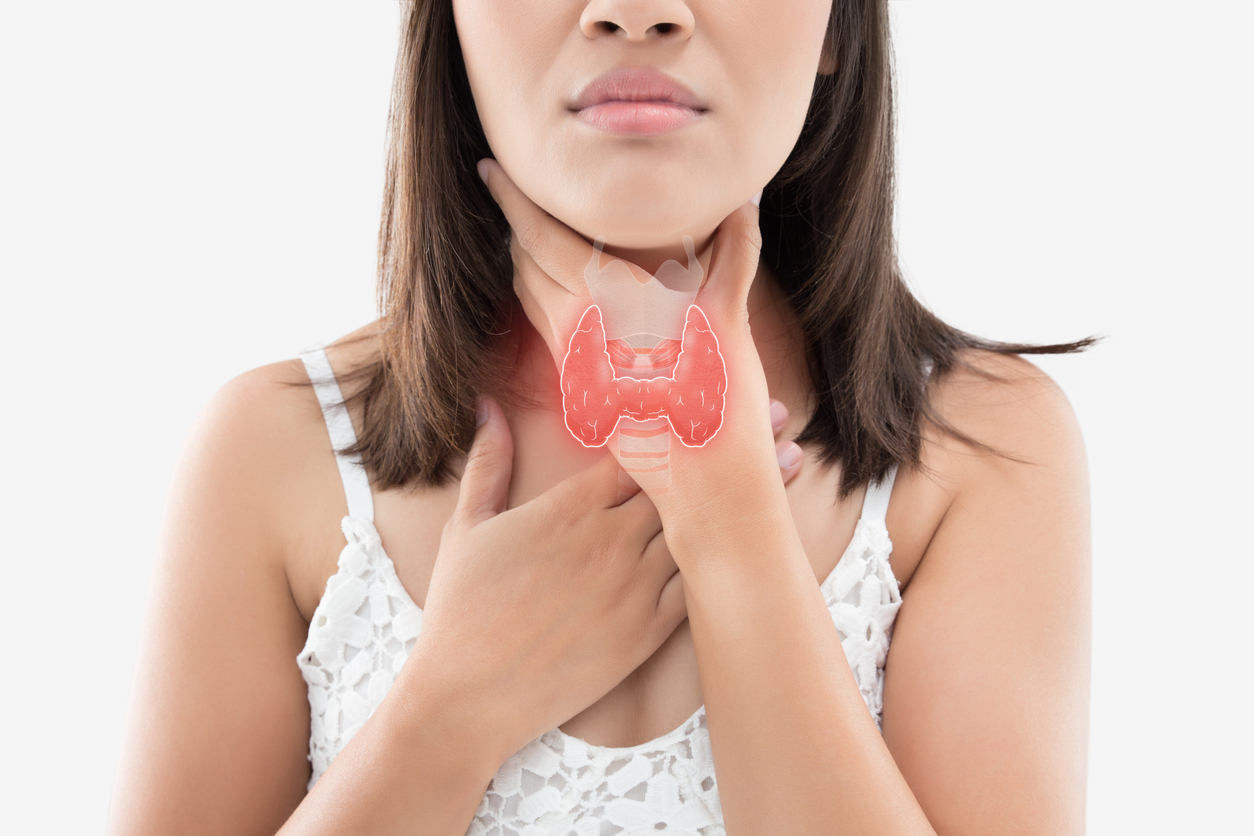
The thyroid gland is a butterfly-shaped gland in your neck that produces two vital hormones (triiodothyronine and thyroxine) that regulate your body temperature, metabolism, and digestion. It also controls essential organs including your heart, brain, liver, kidneys, and skin.
According to the American Thyroid Association, over 60% of people with thyroid disease are unaware of their condition. Furthermore, there are various myths surrounding thyroid disease, which adds to the uncertainty and delays in diagnosis and treatment. So, in order to raise awareness of thyroid illness, we've debunked some of the most popular misconceptions about it.
1. Thyroid disease has clear symptoms
People often believe that if you have thyroid issues, you can quickly tell if something is wrong with your thyroid gland since the symptoms are so obvious. Truth be told, thyroid illness signs might occasionally be imperceptible and simple to miss.
Unfortunately, thyroid illness can be very difficult to detect since the symptoms are vague or overlap. Particularly in women, thyroid diseases are frequently misunderstood. This is due to the potential difficulty in diagnosing thyroid issues, since their symptoms may resemble those of other female-specific illnesses including premenstrual dysphoric disorder, perimenopause, or menopause.
2. Thyroid disease patients will always need to take medications
It depends on what caused your illness. Thyroid issues can occur in certain women before, during, or after pregnancy. The thyroid may self-regulate once the body's hormone levels have stabilized. It's also accurate to say that prescriptions can vary over time. For instance, pregnant women with underactive thyroids may require extra thyroid hormone supplementation.
3. A thyroid lump or nodule indicates malignancy
Only around 5% of thyroid nodules are cancerous on average, with the majority of nodules being benign. Your endocrinologist may encourage you to have a thyroid nodule ultrasound performed, and if further testing is necessary, a nodule needle test may be suggested.
4. Weight loss is impossible with hypothyroidism
Your thyroid regulates your metabolism, which determines how many calories you burn during the day. The pace at which your body can turn food into energy is slowed down by hypothyroidism, which is an underactive thyroid. Even when the thyroid is wrong, most people still need to manage all the other issues that cause weight gain.
It is probable that when one is using medicine to treat hypothyroidism, the basal metabolic rate decreases, making it more challenging to lose weight in comparison to someone who doesn't have hypothyroidism. That is not an excuse, though, not to live a healthy lifestyle.
5. Diet can help you control thyroid disease
Thyroid issues cannot be controlled only by diet. Restoring hormone production to a normal level is possible with thyroid medication. Your general health still depends on eating a balanced diet that is rich in vitamins and minerals.
6. Thyroid issues are just a middle-aged woman's concern
Thyroid disease may really affect both men and women of any age. However, since they have greater estrogen levels than males, women are more likely to develop any thyroid disorder, including hypothyroidism, hyperthyroidism, nodules, and thyroid cancer.
7. Iodine can be used to treat thyroid disease at home
It is true that your thyroid depends on iodine, which is often present in table salt, to function, therefore maintaining a healthy level of the mineral is essential. Additionally, thyroid cancer and hyperactive thyroid diseases are occasionally treated with radioactive iodine treatment (RAI).
However, the majority of people really obtain enough iodine from their regular meals. In fact, consuming too much iodine may exacerbate thyroid issues by causing the body to overproduce thyroid hormone in an effort to handle the excess iodine. So avoid doing it yourself and taking iodine pills without first talking to your doctor.

 Log in with Facebook
Log in with Facebook 





